The main findings on examination and their physiological explanation are as follows:

John Barker
56, Male
Heart failure mechanisms
Heart failure occurs when the heart is unable to pump blood forwards at a sufficient flow to meet the metabolic demands of the body and/or when the heart is only able to pump sufficient blood to meet these metabolic demands with abnormally high cardiac filling pressures.
‘Compensatory mechanisms’ attempt to maintain sufficient blood pressure to perfuse vital organs by compensating for the decrease in cardiac output that occurs:
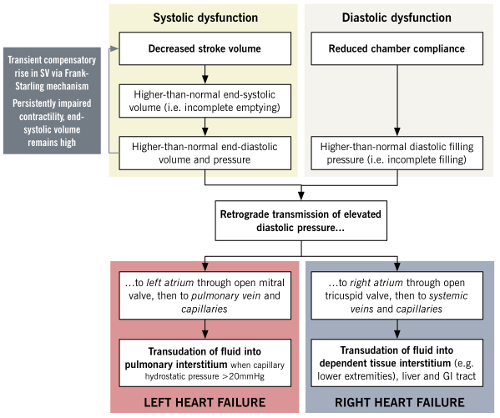
Frank-Starling mechanism: ventricular output increases in relation to preload.
In heart failure, decreased stroke volume results in higher residual diastolic volume which increases stretch of myocardial fibres. This induces greater stroke volume for the subsequent contraction to empty the ventricle and preserve cardiac output.
Limitations of mechanism: At markedy elevated diastolic volumes the stretch of myocardial fibres becomes too great to generate a stronger contraction.
Myocardial hypertrophy: wall stress is estimated from La Place’s relationship in which wall stress is proportional to ventricular pressure and ventricular chamber radius and inversely proportional to ventricular wall thickness.
In heart failure, wall stress increases due to ventricular dilatation and/or the increased pressure required to overcome greater afterload. This leads to hypertrophy of myocytes to maintain contractile force and counteract the wall stress.
Limitations of the mechanism: eventually the ventricle dilates out of proportion to the wall thickness resulting in rapid deterioration of function.
Neurohormonal mechanisms
In heart failure, activation of neurohormonal mechanisms (renin-angiotensin-aldosterone system and antidiuretic hormone) leads to: 1) salt and water retention which increases intravascular volume/preload and maximises stroke volume via the Frank-Starling mechanism; 2) increased systemic vascular resistance which maintains blood pressure despite reduced cardiac output (Blood Pressure = Cardiac Output x Total Peripheral Resistance).
Limitations of the mechanism: increased circulating volume/preload eventually overwhelms the Frank-Starling mechanism; increased total peripheral resistance increases afterload and reduces stroke volume and cardiac output; chronically elevated angiotensin II and aldosterone trigger cytokines which activate macrophages and fibroblasts and results in ‘adverse cardiac remodelling’.
Adrenergic nervous system
In heart failure, activation of baroreceptors triggered by decreased cardiac output activates the sympathetic nervous system which increases heart rate, contractility (Cardiac Output = Heart Rate x Stroke Volume) and total peripheral resistance (Blood Pressure = Cardiac Output x Total Peripheral Resistance).
Limitations of the mechanism: continuous sympathetic activation results in downregulation of β adrenergic receptors and decreased sensitivity to catecholamines; increased heart rate increases metabolic demands and myocardial cell death.
Source: McMasters Pathophysiology Review (http://www.pathophys.org/heartfailure/hf/)
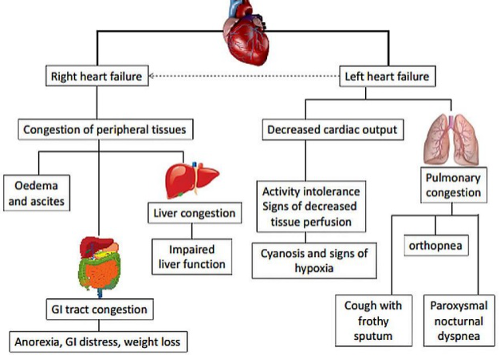
Examination findings
The findings on examination show:
Pansystolic murmur and displaced apex beat
The displaced apex beat reflects left ventricular dilatation. The pansystolic murmur suggests mitral regurgitation. This is likely secondary to loss of mitral valve leaflet coaptation (which occurs when significant left ventricular dilatation stretches the mitral annulus separating the mitral valve apparatus).
According to the La Place relationship, ventricular dilatation and pressure increases lead to increased wall stress and compensatory myocyte hypertrophy. Decompensation occurs when the ventricle eventually dilates out of proportion to the wall thickness.
Crackles at the lung bases
Bilateral fine inspiratory basal crepitations at the lung bases reflects pulmonary oedema (see below: left heart failure).
As described above, reduced ventricular stroke volume increases diastolic volume and left atrial pressure. Salt and water retention increases intravascular volume/preload. Activation of the Frank-Starling mechanism initially compensates by increasing force of contraction. Decompenstion occurs when the Frank-Starling mechanism is overwhelmed. This leads to a further increase in left atrial pressure, lung vasculature congestion, transudation of fluid from the alveolar capillaries and pulmonary oedema.
High left atrial filling pressure may also cause sudden filling of the left ventricle when the mitral valve opens at the beginning of diastole, giving rise to a third heart sound.
Peripheral oedema and elevated JVP
Peripheral oedema, hepatomegaly and elevation of the jugular venous pressure reflect increased right atrial pressure, peripheral vasculature congestion and transudation of fluid into the dependent tissue interstitium (see below: right heart failure).