The Physics of NMR
- Quantum mechanics
- Which nuclei?
- Spin
- Magnetise patients..
- ..then excite them!
- Building blocks
- Getting a signal
- Time to relax...
- NMR occurs because certain atomic nuclei have a property known as spin
- ‘Spin’ (I) is a quantum-mechanical property of the nucleus
- Nuclei with even numbers of protons and neutrons have no spin
- Nuclei with an odd number of protons and/or an odd number of neutrons have integer spin (I = 1, 2, 3…) or half-integer spin (I = 1/2, 3/2, 5/2…)
- Only these nuclei with nonzero spin can be studied using NMR
There is no exact classical analogy for quantum-mechanical spin. A model that works well for many purposes is to picture the nucleus as a solid ball, and a nucleus with spin as rotating on an axis through its centre at a speed that is dependent on the quantum number.
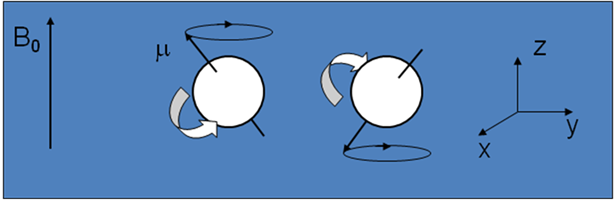
- A nucleus with spin is pictured classically as a spinning ball of charge
- This spinning charge generates a magnetic field, denoted by the magnetic moment, µ
- In an applied magnetic field, B0, according to quantum mechanics the magnetic moment of the 1H nucleus (I=½) adopts one of two orientations (see diagram)
- According to classical electromagnetism, the nuclear magnetic moments experience a torque and precess about the z-axis (defined by B0) at the Larmor frequency, ω0 = γ B0
Here γ is a gyromagnetic ratio, a constant for each nuclear species. For 1H, γ = 42. 57 MHzT-1
The direction of the static field is usually taken to define the z-axis of a coordinate system, and the plane perpendicular to this direction is known as the xy-plane or transverse plane.
Also see: http://www.e-mri.org/nmr/precession.html
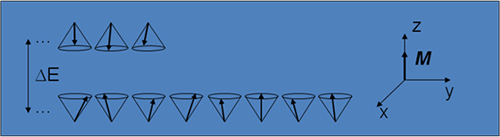
- Nuclei aligned against the field have slightly more energy than those aligned with it, the difference being:
Therefore the two orientations correspond to two quantum-mechanical energy levels.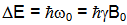
- With a fixed amount of thermal energy available in the system, the lower energy level has a slightly greater population, which gives the sample as a whole a bulk magnetisation, M, lying along the z-axis.
- In the diagram the difference in population has been greatly exaggerated: at room temperature and typical MRI magnetic field strengths there is a excess of only about 5 or 6 nuclei oriented with the field as opposed to against it.
- M is therefore very small, which explains why NMR is such an insensitive experiment and also why manufacturers continually move towards higher field strength magnets (since the population difference depends on ΔΕ which is proportional to B0.
- The orientation of precessing spins in the xy plane is random, so there is no net magnetisation along the x- or y-axes. (In a more strictly accurate quantum-mechanical description, this is a manifestation of Heisenberg’s uncertainty principle.)
-
If an additional magnetic field, B1, is applied which rotates in the xy plane at the same frequency as the bulk magnetisation – i.e. the Larmor frequency ω0 – then a resonance condition occurs.
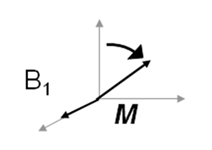
- At the magnetic field strengths used in MRI, ω0 lies in the radiofrequency (RF) part of the electromagnetic spectrum. B1 is therefore provided in the form of a radiowave of the appropriate frequency.
- When this resonance condition is achieved, the bulk magnetisation precesses about the direction of B1 as well as B0, and so it tips (nutates) away from the z axis towards the xy plane.
- Nutation continues until B1 is removed, so the amplitude and duration (t) of B1 can be tailored to nutate M through specific angles α = γB1t.
-
The basic building blocks of simple NMR experiments are 90o and 180o pulses, which are pulses of RF tailored to nutation M through these two angles.
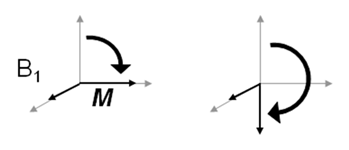
-
Following a 90° pulse, magnetisation is entirely in the transverse (xy) plane
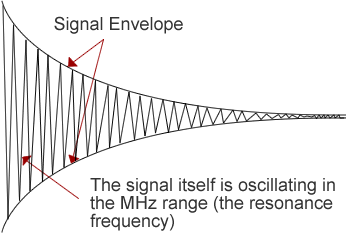
- This magnetisation is precessing in the plane about B0 at the Larmor frequency, at ω0.
- According to Faraday’s law of induction, this changing magnetisation will induces a voltage, and hence a current, in a conductor placed adjacent to the sample.
- In NMR, this ‘conductor’ takes the form of a tuned coil (known as the ‘RF coil’).
- The induced signal, known as a free induction decay (FID), oscillates at ω0 and decays exponentially.
- In practice, it is usual to collect a delayed signal, or ‘echo’, rather than the FID
-
The decay of the FID can be explained by considering what happens to the bulk magnetisation, M, when the RF pulse is switched off.
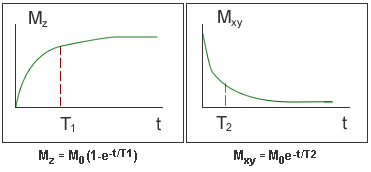
- Over time, M returns to its original orientation along B0 (the z axis) as the transverse magnetisation decays away the end z-magnetisation recovers.
- Recovery of Mz is characterised by the longitudinal relaxation time, known as T1.
- Decay of Mxy is characterised by the transverse relaxation time, known as T2. It is this decay that is seen in the FID envelope.
- The T1 and T2 values of water protons vary between different tissues, and so spins in different tissues recover and decay and different rates.