
Katie Tang
32, Female
Katie tells you more about her recent symptoms.
She has also noticed she has been dropping things with her left hand and that her left leg has felt weak when she walks. She has noticed this yesterday, but it seems to be improving today. However today she has noticed that her left leg is swollen compared to the right leg, and she has no history of injury or trauma.
When you ask Katie about her past medical history, she tells you she is well with no medical conditions or regular medication. When you ask Katie about her social history she tells you that she works in an office and lives with her husband. She also tells you that she does not have any children but that she and her husband are trying for a pregnancy. She tells you that because she has had five miscarriages over the last two years, she has stopped smoking and drinking alcohol.
-
Cryptogenic Stroke and PFO
In ≈40% of patients with acute ischemic stroke, the cause remains undefined. Patent Foramen Ovale (PFO) is a haemodynamically insignificant interatrial communication present in >25% of the adult population. During fetal life, because the lungs do not receive blood flow, blood returning to the right atrium is shunted through the foramen ovale to the left atrium. Postnatally, the foramen ovale closes spontaneously in ≈75% of the population. However, in a portion of adults, by maintaining a direct communication between the right- and left-sided circulation, a PFO can serve as a conduit for paradoxical embolization.
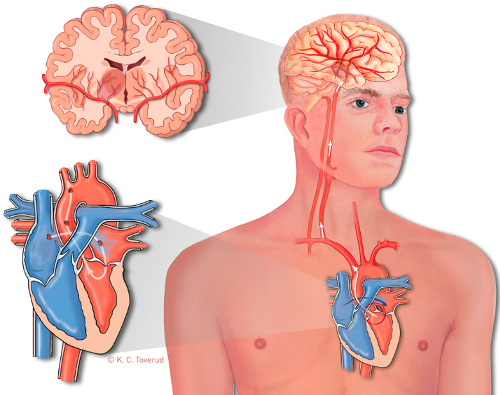
Figure 2
In 1877, Cohnheim described the association of PFO with stroke in a young woman with cerebral arterial embolism. However, it has been difficult to diagnose PFO in vivo until the development of echocardiography and its ability to image the interatrial shunting with an injection of agitated saline (bublles act as an unltrasound contrast agent). With the use of contrast echocardiography, a strong association of cryptogenic stroke with PFO has become evident in patients <55 years of age.
For paradoxical embolization to occur, a source of thrombus is needed. A significant stroke can result from an arterial occlusion by an embolus as small as 1 mm in diameter. Finding of a venous thrombus strengthens the possible role of PFO as a conduit for paradoxical embolization and will affect treatment strategy. Patients with a tendency toward venous thrombus formation have a higher chance of paradoxical embolization, this includes patients with hypercoagulable states. Factor V Leiden mutation causing resistance to activated protein C is the most common risk factor. Factor V Leiden mutation is present in up to 5% of the normal population and is the most common cause of familial thromboembolism. Primary or acquired deficiencies in protein C, protein S, and antithrombin III are other risk factors. The deficiency of these natural anticoagulants is responsible for 10% of venous thrombosis in younger people. In summary, variation in PFO size, right atrial anatomy, hemodynamic conditions, and potential source of an available thrombus all play a part in influencing the chances of paradoxical embolization.
-
Recurrent Miscarriage
Recurrent miscarriage syndrome (RMS) is a common obstetric problem. Proper evaluation can determine the etiology of RMS in almost all women. The most common hemostasis-related cause is a thrombotic disorder, of which the most common is antiphospholipid syndrome (APLS).RMS due to blood protein or platelet defects may come about through either of two mechanisms: (1) disorders associated with a hemorrhagic tendency or (2) defects associated with a thrombotic tendency. Hemorrhagic (bleeding) defects associated with RMS are rare, whereas thrombotic or hypercoagulable/thrombophilic defects are extremely common:
- Lupus anticoagulants and anticardiolipin antibodies (these two comprise the antiphospholipid syndromes that are associated with fetal wastage syndrome)
- Factor V Leiden
- Protein C, S and thrombin deficiency
- Factor XII deficiency
- Dysfibrinogenemias associated with thrombosis
- Heparin cofactor II deficiency
- Fibrinolytic defects associated with thrombosis – Plasminogen deficiency, tissue plasminogen activator [tPA] deficiency, elevated plasminogen activator inhibitor type 1 [PAI-1], and PAI-1 polymorphisms
- Sticky platelet syndrome
- 5,10-methyltetrahydrofolate reductase (5,10-MTHFR) mutations
- Prothrombin G20210A gene mutation
- Hyperhomocysteinemia
- Lipoprotein(a) elevation
- Immune vasculitis