The main findings on examination and their physiological explanation are as follows:
Bradycardia
Adam exhibits a resting bradycardia, defined as a heart rate of <60 beats per minute. This is almost certainly due to regular exercise training and the level of athletic activity he does. Regular participation in exercise stimulates bradycardia and this is therefore a common finding in fit, young, athletic people. The mechanism is thought to be due to a combination of factors including parasympathetic activation, decreased responsiveness to beta-adrenergic stimulation and decreased intrinsic heart rate.
Bradycardia is easily overcome with exercise, suggesting a vagal aetiology, although evidence exists from chemically denervated hearts that sinus pacemaker cells may be influenced by athletic conditioning independent of vagal input. More recent work in mice after blockade of the autonomic nervous system and in vitro in denervated sinus node cells suggest that the mechanism may include remodelling of pacemaker cells and in particular, downregulation of hyperpolarisation-activated cyclic nucleotide-gated (HCN), Na+, Ca2+ and K+ channels.
Pulse character
A jerky pulse character occurs when there is rapid ejection of blood from a left ventricle which has outflow tract obstruction. It is characterised by a rapid upstroke from vigorous contraction of a hypertrophied ventricle; as the volume of the left ventricle decreases and the blood suddenly meets the obstruction in the left ventricular outflow tract, this results in a rapid fall in arterial pressure and the “jerky” character to the pulse.
A regularly irregular pulse can occur in a number of different situations, but in this case appears to be due to pulsus bigeminus, also called a bigeminal pulse, secondary to regular ectopic beats after every sinus beat (ventricular bigemeny). In ventricular bigemeny, there is a premature ventricular ectopic beat following every sinus beat. The sinus beat is felt as a normal volume beat and the ectopic may be felt as a weaker beat. The pulse is not regular because each ectopic beat is premature. Ventricular trigeminy may also cause a regularly irregular pulse, but this time there is an ectopic after every second heartbeat rather than after every heartbeat as in bigemeny. Pulsus bigeminus should not be confused with pulsus alternas (see tutorial on pulse character below). The other classic cause of a regularly irregular pulse is Mobitz type 1 second-degree atrioventricular block (Wenckebach) where the PR interval gradually prolongs and eventually results in a non-conducted p-wave (felt as a dropped heartbeat) in a regular cycle. Of course, the classic example of an irregularly irregular pulse is atrial fibrillation.
A double apical impulse apex beat occurs when two apical pulsations are felt with every heartbeat. It occurs because of a palpable atrial impulse which itself is due to forceful atrial contraction against a stiffened ventricle. This atrial impulse is, in essence, a palpable 4th heart sound (see below).
A 4th heart sound is always abnormal and occurs in late ventricular diastole, just before the 1st heart sound (i.e. just before closure of the atrioventricular valves). It represents atrial contraction against a ventricle with reduced compliance made stiff by any cause such as hypertension or aortic stenosis. It sounds like “da-lub dub” or “Ten-nes-see”.
An example of a 4th heart sound can be heard here...
Listen and compare with ...
Ejection systolic murmur
An ejection systolic murmur usually originates from the left ventricular outflow tract and rises and falls in intensity as intraventricular pressures rise and fall during ventricular contraction and relaxation, creating a “whooshing” sound. It represents turbulent blood flow out of a ventricle, either in the outflow tract or across the aortic or pulmonary valve. Because of the rise and fall in intensity during the cardiac cycle, an ejection systolic murmur is also described as a “crescendo-decrescendo” murmur and often represented graphically as follows (Figure 5):
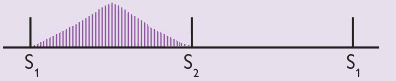
Although ejection systolic murmurs can be innocent and found in children, healthy young adults and high-cardiac output states such as tachycardia and pregnancy, pathological causes include fever, aortic stenosis and sclerosis, pulmonary stenosis and hypertrophic cardiomyopathy with obstruction.
Certain manoeuvres can help differentiate different causes of an ejection systolic murmur. Manoeuvres which cause a decrease in preload including standing up from a squatting position and the Valsalva manoeuvre. In the former, standing causes pooling of blood in the legs due to gravity and reduction of venous flow to the heart, resulting in a decrease in ventricular filling. With the Valsalva, intrathoracic pressure is increased and impedes the return of systemic blood to the heart, again resulting in a decrease in ventricular filling. Both these manoeuvres cause less blood to be ejected through the aortic valve and therefore in aortic stenosis, they cause a decrease in the intensity of the murmur.
In hypertrophic cardiomyopathy (HCM) with obstruction, the anatomic abnormality is hypertrophy of the septum. This thickened septum causes 2 main effects. Firstly, it causes narrowing of the left ventricular outflow tract and physical obstruction of blood flow out of the heart. Secondly, the narrowing causes increased velocity of blood flow through the left ventricular outflow tract and produces a Venturi effect which sucks in the anterior and sometimes posterior mitral valve leaflets into the outflow tract, further exacerbating the outflow tract obstruction. The key thing to remember is that unlike aortic stenosis, where the physical obstruction to blood flow through the narrowed aortic valve is relatively fixed, the obstruction in HCM is dynamic. In other words, it can change with different loading conditions. Any manoeuvre that decreases venous return to the heart (such as standing from a squatting position and the Valsalva) results in decreased left ventricular end-diastolic volume. This decrease in left ventricular end-diastolic volume worsens the outflow tract narrowing by allowing further approximation of the hypertrophied septum to the mitral valve apparatus, worsening the outflow tract obstruction during systole, and therefore increasing the intensity of the murmur, in contrast to aortic stenosis.
So, in summary, the Valsalva manoeuvre or standing from a squatting positing increase the intensity of an ejection systolic murmur due to hypertrophic cardiomyopathy with obstruction and decrease the intensity of an ejection systolic murmur due to aortic stenosis.
An example of an ejection systolic murmur can be heard here...
(Compare with a pansystolic murmur below, so you get used to your ears differentiating between the two).
Pansystolic murmur
A pansystolic murmur is of uniform intensity throughout systole and merges with the second heart sound. It is usually due to backflow of blood from a ventricle into an atrium – in other words, mitral regurgitation or tricuspid regurgitation. Other causes include a ventricular septal defect. Pansystolic murmurs are almost always pathological. As the intensity of the murmur is uniform throughout ventricular systole (in contrast to ejection systolic murmurs), pansystolic murmurs are often represented graphically as follows:
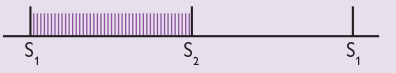
An example of a pansystolic murmur can be heard here...
(Compare with an ejection systolic murmur above, so you get used to your ears differentiating between the two).
Cardiac cycle
Finally, remember: it is useful to think about and keep the cardiac cycle in mind or have it to hand as a reference when listening to and learning about heart sounds and murmurs (see Figure 7 below).
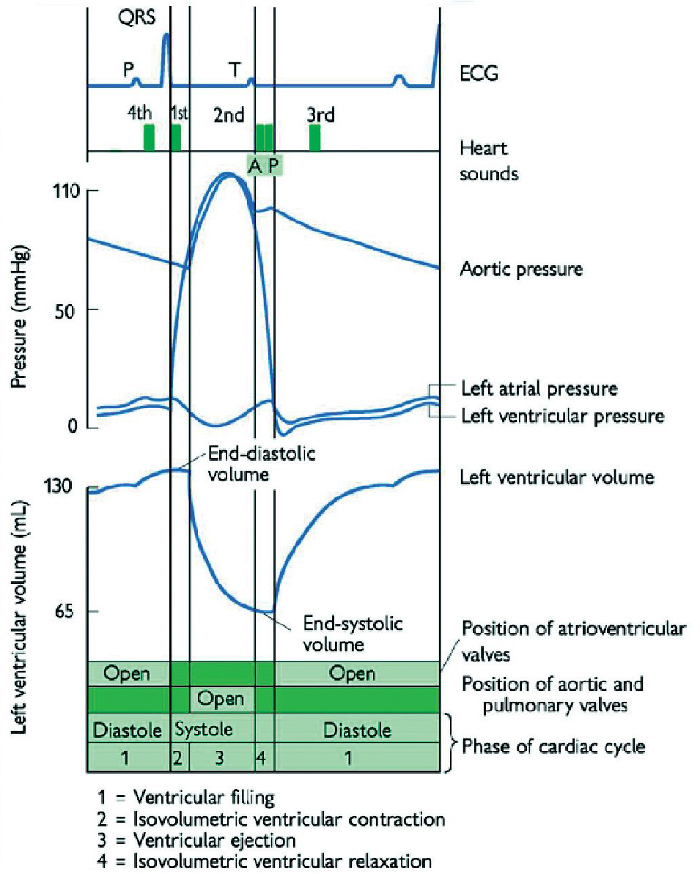
SOURCE: Taken from the Oxford Handbook of Clinical Medicine, 10th Edition, Oxford University Press 2017.