
Adam Smith
37, Male
Click on the tabs below (and click headings to open and close the subpanels) to view the results and interpretation of the immediate investigations.
12 lead ECG
Result:
Explanation
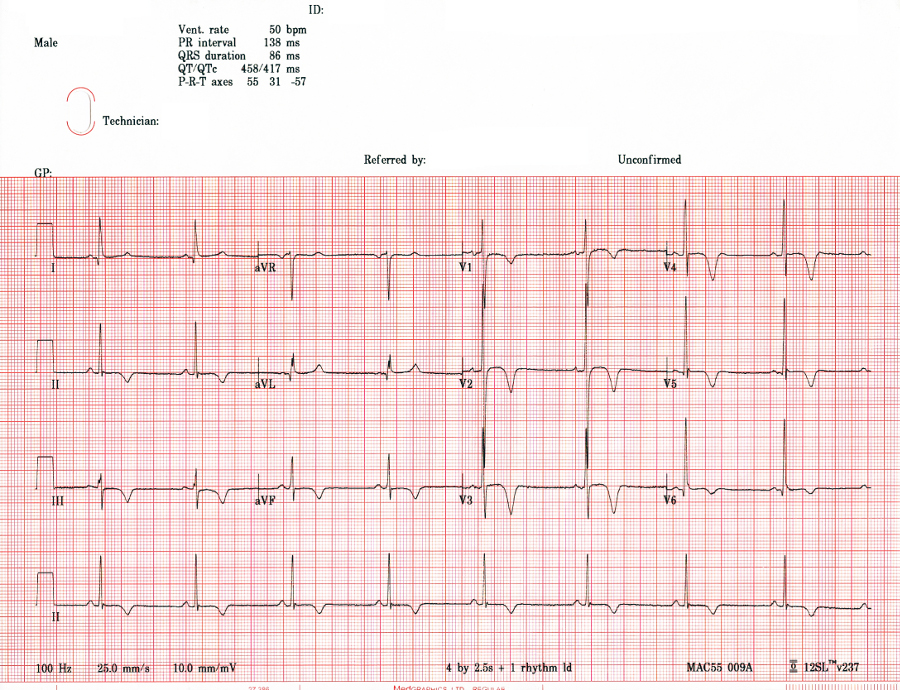
The 12-lead ECG (Figure 9) shows a resting sinus bradycardia at a rate of 50 beats per minute though no evidence of the intermittent ventricular ectopics that were felt occasionally on examination as pulsus bigeminus. The main features are as follows:
- Sokolow-Lyon voltage criteria for left ventricular hypertrophy, namely the sum of the S wave in V1 and the R wave in V5 or V6 (whichever is bigger) is ≥35mm (3.5mV)
- Deep (≥2mm or 0.2mV) T-wave inversion across the anterior leads (V1-V4), some of the lateral leads (V5 and V6, though not I and aVL) and the inferior leads (II, III and aVF)
- Fixed resting ST-segment depression of 0.5-1mm in the inferior leads II and aVF
- A pathological Q-wave in aVL. A Q-wave is defined as pathological if its size (downward deflection) is ≥25% the size of the ensuing R-wave. Here, the Q-wave is just over 1.5mm and the R-wave following it is 5.5mm. So the Q-wave is (1.5/5.5)x100 = 27% of the R-wave.
- By this definition, there are also small non-pathological Q-waves in leads I and V6
- There is also convex ST-segment elevation in V2 which is likely benign (“high take-off”) and may be related to exercise training or be a normal finding in young, thin, fit individuals
These features are all suggestive of pathological left ventricular hypertrophy which may be due to a number of different causes. Of course, the differential diagnosis would also include cardiac ischaemia; however, this is less likely given the fixed and non-dynamic nature of the ECG changes, their widespread extent (which would imply several coronary territories and would be very unusual in a man of Adam’s age) and, most importantly, the clinical picture: Adam is now looking well and has no chest pain. Furthermore, his chest pain did not sound typical of angina.
(Remember – it is always crucial to interpret the 12-lead ECG in the context of the clinical picture and patient in front of you).
Full blood count
Explanation:
Adam has been experiencing breathlessness on exertion which can sometimes be caused by severe anaemia. Therefore a full blood count should be performed. Anaemia can also trigger angina in those with significant coronary artery disease; however, Adam’s chest pain sounded atypical for angina.
Results & Explanation
| Investigation Name | Investigation Result | Normal Range | Units |
|---|---|---|---|
| Haemoglobin | 155 | 115-165 | g/L |
| White cell count | 9.0 | 4-11 | x109/L |
| Neutrophils | 4.5 | 1.5-7.0 | x109/L |
| Platelets | 209 | 150-400 | x109/L |
These results are within normal range.
Renal function
Rationale:
Given Adam’s symptoms of breathlessness, palpitation and syncope, it would be important to know that his renal function is normal as well as his electrolytes. The latter in particular can cause syncope and palpitation if abnormal, for example hyper- or hypokalaemia.
Results & Explanation
| Investigation Name | Investigation Result | Normal Range | Units |
|---|---|---|---|
| Sodium | 140 | 137 - 145 | mmol/L |
| Potassium | 4.1 | 3.5 - 4.9 | mmol/L |
| Urea | 4.7 | 2.5 - 7.5 | mmol/L |
| Creatinine | 63 | 60 - 10 | µmol/L |
Adam’s renal function and electrolytes are both normal.
C-Reactive Protein (CRP)
Rationale:
The CRP will be elevated in any inflammatory condition, and particularly in infection. It is an acute phase protein produced predominantly by the liver in response to inflammatory cytokines. As Adam has been feeling breathless recently, this is a reasonable test to perform, although he has not been experiencing other infective symptoms (such a fever, cough, productive sputum etc).
Results & Explanation
| Investigation Name | Investigation Result | Normal Range | Units |
|---|---|---|---|
| CRP | 1 | 0 - 4 | mg/L |
This CRP level is normal.
Troponin
Rationale:
Adam has presented with chest pain and has been experiencing palpitation and syncope. In addition, he has a highly abnormal ECG. Although Adam’s young age and atypical symptoms make an acute myocardial infarction unlikely, he has a highly abnormal ECG and therefore a troponin level, both initial and at 4 hours, would be warranted. The reasoning behind this is given below where we look at the result.
Results & Explanation
| Investigation Name | Investigation Result | Normal Range | Units |
|---|---|---|---|
| Troponin 1 hour | 41 | < 14 | ng/L |
| Troponin 4 hour | 39 | < 14 | ng/L |
We find that Adam’s initial troponin level is elevated and a repeat 4 hours later is roughly the same. His ECG remains completely unchanged and is the same as shown in Figure 9 above. He remains pain free.
Why is the troponin elevated if Adam is not having a myocardial infarction and no symptoms of ischaemia? Troponin is found in cardiac myocytes and leaks into the blood stream after myocyte cell damage or death (leading to necrosis; Figure 10).
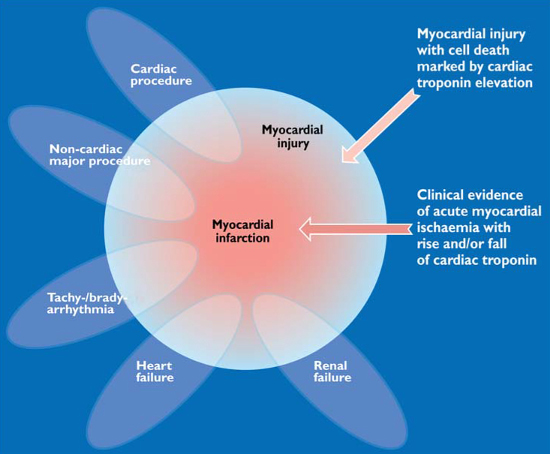
It is important to remember that many different conditions and stresses on the heart can cause an elevation in the troponin level (Figure 11). An elevation in the troponin level means that there has been recent myocardial cell damage and injury, but this can occur through a number of different mechanisms and does NOT necessarily mean that there has been ischaemic myocardial cell damage from a myocardial infarction. A classical myocardial infarction is diagnosed if there is clinical evidence of acute myocardial ischaemia accompanying the rise and/or fall of cardiac troponin, but myocardial injury may occur through several non-ischaemic mechanisms, as illustrated in Figure 11.
SOURCE: From Alpert et al., European Heart Journal, (2012) 33, 2551–2567.
Figure 12 below lists the different mechanisms which may eventually lead to myocardial cell injury and an elevation in the troponin level. As you can see from this list, a “traditional” myocardial infarction where there is coronary artery plaque rupture and subsequent occlusion to blood flow due to intraluminal coronary artery thrombus formation is but one of many different mechanisms that can lead to myocyte cell death. Indeed, the list is diverse, from myocardial infarction all the way down to strenuous exercise.
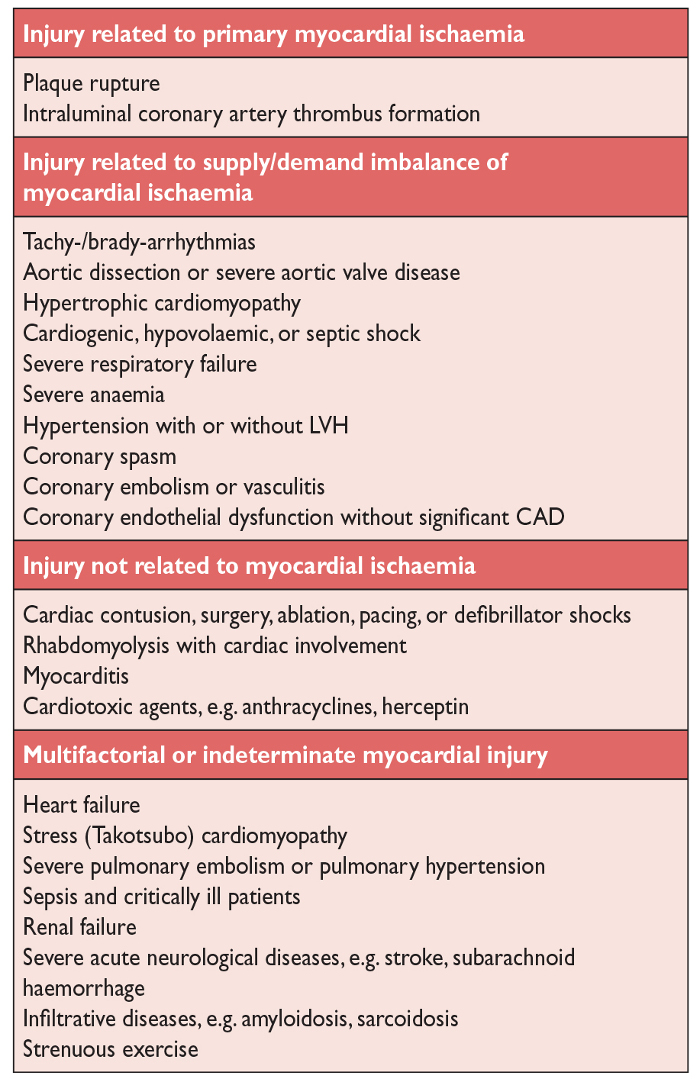
SOURCE: From Alpert et al., European Heart Journal, (2012) 33, 2551–2567.
When we talk about an acute coronary syndrome (ACS), this is a clinical description and refers to any group of clinical symptoms compatible with acute myocardial ischemia, from unstable angina, to a non-ST-segment elevation myocardial infarction (NSTEMI), through to a ST-segment elevation myocardial infarction (STEMI). Myocardial infarctions themselves can be divided up into different types, according to the primary mechanism of action or clinical circumstance. We will not go into myocardial infarction in detail in this case scenario, as it is covered in another module. However, Figure 13 summarises the categories of myocardial infarction.

SOURCE: Taken from Chapman AR, et al. Heart 2017;103:10–18.
However, ACS is but one of a surprisingly large range of conditions that can cause an elevation in troponin levels.
Because of this and to help simplify things, we can think of troponin elevations in 3 distinct categories rather than thinking of them in solely in terms of ACS and myocardial infarction:
- Primary ischaemic cardiac injury: this refers to troponin elevations caused by ACS, usually due to rupture of an atheromatous plaque which leads to platelet aggregation (which is why antiplatelet agents such as aspirin are so crucial in the treatment of ACS) and “white” thrombus. Subsequent progression to activation of the clotting cascade results in fibrin clot formation (“red” thrombus) with vessel occlusion (which is why heparin is used in ACS), and subsequent myocardial necrosis in the area supplied by the occluded artery. A Type 1 myocardial infarction would fit into this category (see ACS Case Scenario from Supporting Life block).
- Secondary ischaemic cardiac injury: this refers to cardiac ischaemia caused by any mechanism other than plaque rupture that imposes an oxygen supply-demand mismatch on the myocardium. This would include Type 2 myocardial infarction, but also Type 4 and Type 5 myocardial infarctions.
- Non-ischaemic cardiac injury: this refers to cardiac cell injury that arises due to direct myocardial involvement in the pathological process itself. An example would by myocarditis from any cause (such as a virus or inflammation cause by cardiac sarcoid) or direct trauma to the heart in a stabbing.
These various causes are summarised in Figure 14 below:
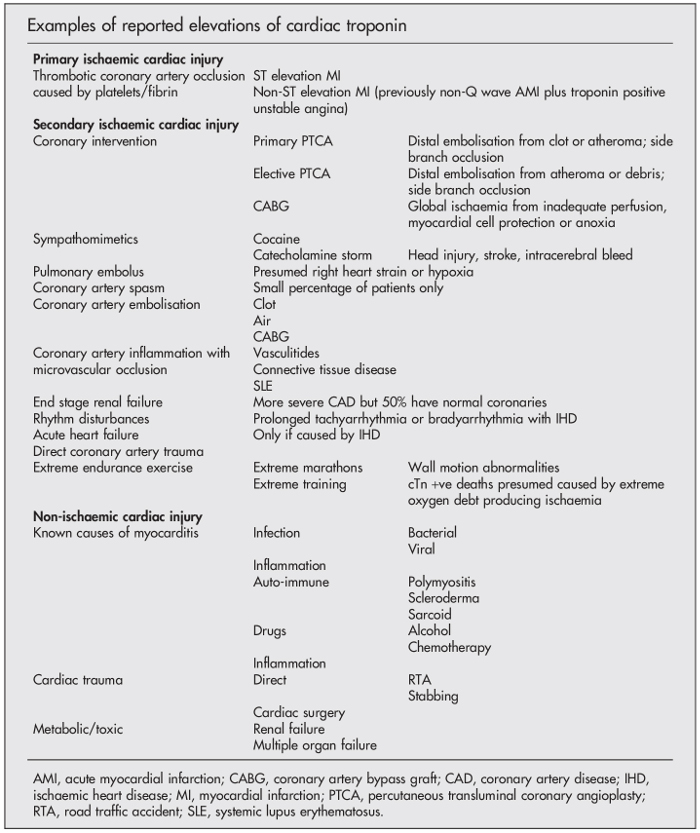
Therefore in Adam’s case, he may have secondary ischaemic cardiac injury from an arrhythmia given his symptom of palpitation, or non-ischaemic cardiac injury. The other possibility given that we suspect pathological left ventricular hypertrophy would be secondary ischaemic cardiac injury due to a mismatch in oxygen supply and demand while he was sprinting up the stairs because of a very hypertrophied ventricle (typically the heart muscle grows more than the blood vessels leading to “capillary rarefaction”). He has no clinical features of an ACS.
D-dimer
Rationale:
Adam has presented with chest pain which he described at times as sharp, as well as breathlessness. In addition, he has also noticed breathlessness on exertion over the last few months. Therefore pulmonary embolic disease may be a possibility. This is less likely given his oxygen saturations of 99% on air, but these do not rule out pulmonary embolism (PE) on their own.
One way of assessing the pre-test probability of Adam having a PE is to use the modified two-level Wells Score shown in Figure 15 below and PE case scenario:
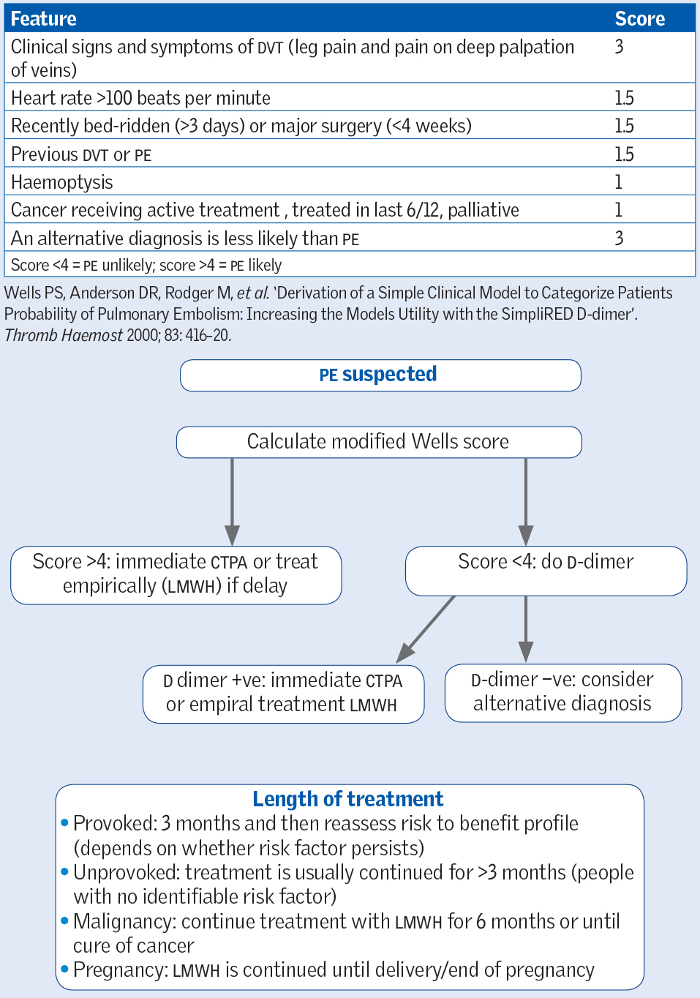
SOURCE: Taken from the Oxford Handbook of Clinical Medicine, 10th Edition, OUP 2017.
Coagulation occurs when proteins of the coagulation cascade are activated. A by-product of this cascade is the production of D-dimers, which are fibrin degradation products and not usually present in the bloodstream. Therefore measuring the D-dimer level in the blood can be very useful in helping to rule out thromboembolism such as a deep vein thrombosis (DVT) or PE: a negative D-dimer makes it very unlikely that the patient has thromboembolism given extremely high negative-predictive value of a negative D-dimer result in someone with a low pre-test probability (the Wells score helps determine this pre-test probability). Conversely, however, there are a number of circumstances that can lead to a positive D-dimer level other than DVT and PE and therefore a positive level cannot be used to diagnose a PE or DVT.
Results & Explanation
| Investigation Name | Investigation Result | Normal Range | Units |
|---|---|---|---|
| D-Dimer | negative |
Adam has a very low pre-test probability of PE and his D-dimer is negative, making pulmonary embolism as a cause of his presentation extremely unlikely.
Thyroid function
Rationale:
Adam’s history of palpitation is suggestive of an arrhythmia and therefore it would be important to check thyroid function. Thyroid hormones have both a positive chronotropic and inotropic effect on the myocardium and thyrotoxicosis can lead to arrhythmias, most commonly atrial fibrillation, possibly through increased triggered activity of atrial tissue in around the pulmonary veins.
The physiology of thyroid hormone production is illustrated in Figure 16 below; thyroid hormones are released from the thyroid gland in response to TSH from the pituitary. Therefore TSH levels are normally measured and, if within the normal range, indicate normal thyroid function.
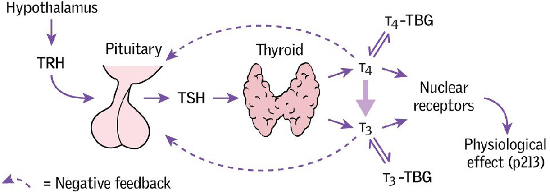
SOURCE: Taken from the Oxford Handbook of Clinical Medicine, 10th Edition, OUP 2017.
Results & Explanation
| Investigation Name | Investigation Result | Normal Range | Units |
|---|---|---|---|
| TSH | 3.3 | 0.27 - 4.2 | mlU/L |
Adam’s thyroid stimulating hormone (TSH) is within normal range.
Lipid profile
Rationale:
Although we do not have a high suspicion of ACS and therefore coronary disease in Adam, and he has no peripheral stigmata of hyperlipidaemia such as xanthelasma, he does have a family history of sudden death at a young age which may be due to pre-mature coronary atherosclerosis (the details of his uncle’s death are not clear). One of the causes for this could be a familial hypercholesterolemia or hyperlipidaemia syndrome, and so it would be reasonable to check a lipid profile (although to be accurate, a fasting level must be taken).
Results & Explanation
| Investigation Name | Investigation Result | Normal Range | Units |
|---|---|---|---|
| Total Cholesterol | 3.9 | < 5 | mmol/L |
Adam’s cholesterol level is well within the normal range and, in fact, quite low.
Chest X-ray
Rationale:
Adam has been experiencing breathlessness on exertion for a while and has also presented with acute breathlessness and chest pain which is sharp at times. Therefore a chest X-ray is important to rule out lung pathology such as a pneumothorax, although given the pain has settled and he is no longer breathless, this makes a pneumothorax unlikely. He may nevertheless have a small apical pneumothorax which could be seen on chest X-ray. A pneumothorax may often be spontaneous in young, thin men. Risk factors include asthma, COPD, TB, pneumonia, chest trauma and connective tissue disorders such as Marfan and Ehlers-Danlos syndromes. Adam’s chest X-ray is shown in Figure 17 below.
Results & Explanation
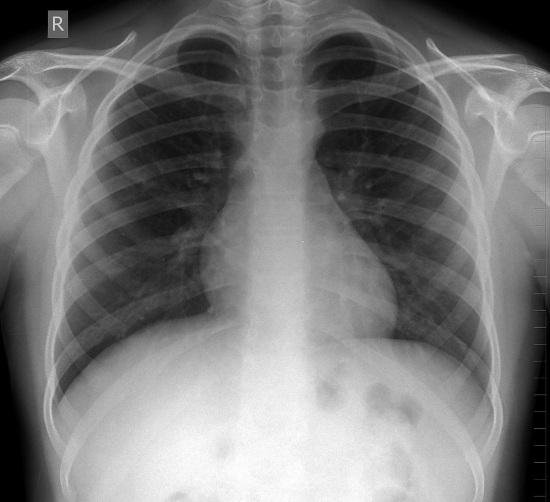
Adam has a normal chest x-ray with clear lung fields, no pneumothorax, no rib fractures, no lung masses / lesions, no hilar lymphadenopathy and a normal cardiothoracic ratio.