
Adam Smith
37, Male
Although we have come across some examples of different types of pulse character, apex beat, heart sounds and murmurs in this case so far, it is worth revising the other findings that examination of these parts of the cardiovascular examination may reveal and their physiological explanation.
Tutorial on pulse character, apex beat, heart sounds, and murmurs (unrelated to this case)
-
PULSE CHARACTER
Much can be learned by palpation of a major arterial pulse such as the carotid artery, and specific conditions result in specific findings (some of which illustrated graphically in Figure 8). This can give vital clues to the underlying pathology even before auscultation of the heart has taken place. Apart from the jerky pulse described in this case, several other characteristic patters are also recognised:
Slow rising pulse: occurs in aortic stenosis. Because of obstruction to blood flow from the narrowed aortic valve, the pulse is not only low in volume but also slow in rising to a peak due to the delay in ejecting blood through the narrowed valve. Hence the term slow rising (see Figure 8) - or pulse parvus (weak/small – low volume) et tardus (delayed – slow rising) for those who prefer Latin!
Collapsing pulse: also known as a water hammer pulse (named after a Victorian / Irish children’s toy called a “water hammer”) or Corrigan’s pulse, this characteristically occurs in aortic regurgitation. It is a large volume pulse characterised by a short duration and a forceful brisk rise with a subsequent brisk fall (see Figure 8). The sensation felt on the fingers is like the thud of a child’s water hammer toy and the quick fall is why it is also called collapsing. The large volume and brisk upstroke occurs because aortic regurgitation causes a volume-loaded left ventricle and therefore a high stroke volume and systolic pressure. The brisk fall (“collapsing” nature) of the pulse is caused by both blood leaking back into the left ventricle from the aorta during diastole, but also as a result of decreased systemic vascular resistance – the latter is caused by baroreceptors in the aortic arch which are stimulated by the increased cardiac output, resulting in reflex vasodilatation of the peripheral vessels into which blood flows rapidly. The brisk fall of the collapsing pulse can be accentuated by using gravity and sharply lifting the patient’s arm up above their head (after asking if they have pain in their shoulder, of course!)
Pulsus bisiferens: describes a high-volume pulse with 2 peaks rather than one (see Figure 8). Both peaks occur in systole and are part of the same pulse wave (see Figure 8). Pulsus bisiferens occurs in mixed aortic valve disease with stenosis and regurgitation. The first peak is caused by the large volume of blood ejected in systole and second peak due to elastic recoil in the arteries. The dip is thought to be caused by the Venturi effect.
Bounding pulse: this can be caused by carbon dioxide retention, liver failure and sepsis. In each situation, the mechanism is a combination of vasodilatation, tachycardia and a high output circulation and usually signifies a high pulse pressure (i.e. difference between systolic and diastolic blood pressure).
Pulsus alternans: describes alternating strong and weak heart beats and is a feature of severe myocardial failure and the prolonged recovery time of a damaged myocardium. a very poor prognosis.
Pulsus paradoxus: this was first described by the German physician Adolf Kussmaul and can be a confusing term so requires more explanation. Pulsus paradoxus is a misnomer because it is actually due to an exaggeration of normal physiology. In a normal healthy subject, during inspiration, because of an increase in negative thoracic pressure, blood is sucked into the right side of the heart. At the same time, this negative intrathoracic pressure also causes stretching of the lungs (which is of course what sucks air into them) but also expands the compliant lung vasculature. This means that some blood momentarily pools in the lung vasculature, and results in decreased pulmonary venous return to the left atrium. At the same time, the increased venous return to the right side of the heart also momentarily causes the right heart to expand and the interventricular septum to bulge into the left side of the heart, further compromising left-sided filling. The overall result is that normally during inspiration, your systolic blood pressure falls.
In normal healthy people, this fall is <10mmHg and can be measured using a sphygmomanometer by inflating the cuff above systolic blood pressure and then lowering the pressure very slowly until the first Korotkoff sound is heard - this first sound is only heard during expiration. If the cuff pressure is then slowly lowered further to the highest pressure at which Korokoff sounds are heard with each beat, this is the pressure at which sounds are being heard with both inspiration and expiration. The difference between the two figures is the pulsus measurement and should be <10mmHg. You can even feel this on yourself if you palpate your radial artery during deep inspiration – there is a small but definite decrease in the pulse amplitude.
Pulsus paradoxus is the term used to describe the clinical situation where the inspiratory fall in systolic blood pressure is >10mmHg. It occurs in the setting of several conditions, most commonly acute pericardial tamponade but can also be a feature of acute severe asthma with an FEV1 of <0.7L, as well as shock and acute pulmonary embolism. Each of these conditions results in exaggeration of the reduction in left ventricular filling that usually occurs with inspiration. With pericardial tamponade, the pericardial space is constrained due to the effusion; thus the normal bulging of the RV into the LV is exaggerated and limits left ventricular filling more than usual. There is also probably more pooling of blood in the pulmonary vasculature.
So what is the “paradox” in pulsus paradoxus? This actually refers to the finding that when pulsus is present, the peripheral pulse may disappear despite the simultaneous presence of the central heartbeat and ventricular contraction. As Kussmaul phrased it: “The pulse was simultaneously slight and irregular, disappearing during inspiration and returning upon expiration.” Thus the disappearance of the peripheral pulse despite the heart beating is the paradox in pulsus paradoxus.
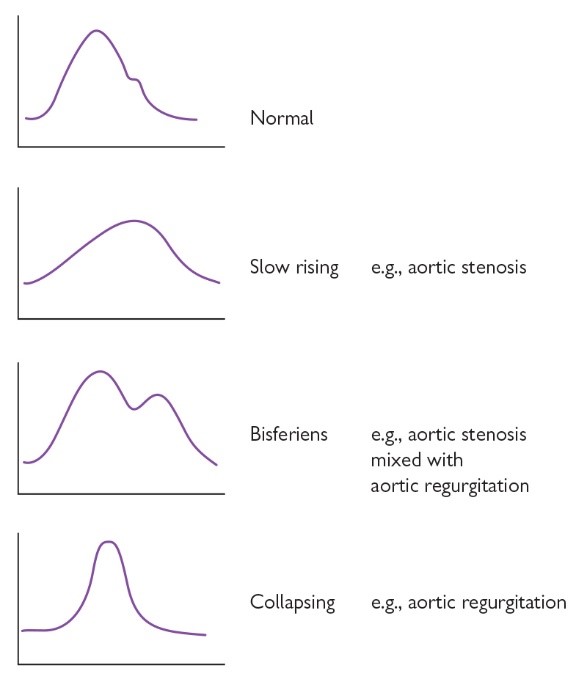
Fig 8. Graphical representation of some of the different pulse characters that can be palpated on examination of a major arterial pulse. These may often point to a specific diagnosis.
SOURCE: Oxford Handbook of Clinical Examination and Practical Skills, Oxford University Press, 2014. -
APEX BEAT
As with palpation of a major artery, information can be gained about underlying pathology by palpation of the apex beat and examining its character. Of course, the position of the apex beat may also be displaced from the normal position (5th intercostal space, mid-clavicular line) due to cardiac pathology (for example, lateral displacement in conditions which cause left ventricular chamber enlargement). However, here we are talking about the apex character. As well as the double apical impulse described and explained above, a number of other apical characteristics are described:
Heaving/Sustained apex: describes the apex beat in a pressure loaded ventricle, usually due to outflow tract obstruction such as aortic stenosis or other forms of pressure overload such as hypertension which cause left ventricular hypertrophy. The apex beat is usually well localised (i.e. palpable over a small area), not displaced, and feels forceful (“heaving”) and sustained against the hand.
Thrusting/Dynamic apex: describes the apex beat in a volume loaded, hyperdynamic ventricle, for example due to valvular regurgitation (most commonly mitral or aortic regurgitation). Ventricular dilatation may have also occurred, causing inferior and lateral displacement of the apex beat. The apex feels diffuse (i.e. palpable over a larger area), and vigorous but non-sustained against the hand.
Tapping apex beat: describes the apex beat in mitral stenosis; the sudden, brief tapping sensation is essentially a palpable 1st heart sound which is accentuated in mitral stenosis because the narrowed valve orifice limits ventricular filling. As a result, there is no gradual decrease in flow towards the end of diastole. The valve leaflets are, therefore, at their maximum excursion at the end of diastole, and so shut rapidly leading to a loud S1 which is palpable at the apex. The first sound is also amplified by the increase in left atrial pressure that accompanies significant mitral stenosis. As a result the mitral valve closes slightly later in systole when the rate of change of pressure is higher, effectively slamming the valve shut.
Absent / weak: the apex may be difficult to feel or indeed absent in a number of conditions such as emphysema, obesity or pericardial effusion. Don’t forget dextrocardia! If no apex beat is felt in the normal position, check on the patient’s right-hand side! It can be difficult to feel the apex beat in a healthy person at rest.
-
HEART SOUNDS
The normal first (S1) and second (S2) heart sounds are usually clear, and represent closure of the mitral and tricuspid (S1) and aortic and pulmonary (S2) valves.
The 1st heart sound (S1) is loud in mitral stenosis, as described in the section on apex beat above. It is also loud if diastolic filling time is shortened, for example if the PR interval is short and with tachycardia. S1 is soft if diastolic filling time is prolonged (for example first degree heart block or if the mitral valve leaflets fail to close properly such as in mitral regurgitation).
The 2nd heart sound (S2) is loud in tachycardia and hypertension, but clinically this is of limited use. Most useful clinically, however, is the intensity of S2 in aortic stenosis where a very soft or even absent S2, in combination with other clinical features (e.g. signs of heart failure and a slow rising pulse) point toward severe or even critical aortic stenosis
A 3rd heart sound (S3) occurs just after S2 and also described as a “triple” or “gallop” rhythm, said to sound like “da-da-dum” or “ken-tuck-y”. It is low pitched so usually best heard with the bell of the stethoscope. It can be physiological but is usually pathological above the age of 30. A loud S3 occurs in either a dilated left ventricular due to rapid ventricular filling (such as mitral regurgitation or a ventricular septal defect) or in a poor left ventricle. It occurs as a higher pitched “pericardial knock” in constrictive pericarditis or restrictive cardiomyopathy.
We have covered the 4th heart sound (S4) above – this represents atrial contraction against a stiffened ventricle from any cause.
-
MURMURS
Murmurs are distinct from heart sounds and usually represent turbulent blood flow in the heart, across the heart valves, and occasionally in other parts of the vasculature. Before auscultation of the heart, always consider the patients symptoms and other signs you have identified up till this point in your history and examination and think to yourself what you might expect to hear. For example, if you have felt a slow rising pulse, then you might expect to hear the ejection systolic murmur and the quiet S2 of severe aortic stenosis.
TWe have already described 2 types of systolic murmur, the ejection systolic murmur and the pansystolic murmur, above as they are related to this case. However, you should also know about and be able to recognise one more type of systolic murmur and several diastolic murmurs (remember – it is always useful to relate these to the cardiac cycle: see Figure 7 on previous screen).
Late systolic murmur: this typically occurs in mitral valve prolapse and by definition occurs late in systole with an audible gap between S1 and the murmur. In mitral valve prolapse the mitral valve first closes and then prolapses backwards after; it is only after the prolapse that blood starts leaking back into the left atrium, which is why the murmur is delayed and not holosystolic (pansystolic) like in typical mitral regurgitation. The moment of valve leaflet prolapse may also be heard just before the start of the murmur as a mid-systolic click, which can be mistaken for a heart sound.
Early diastolic murmur: this is usually due to aortic (or pulmonary) regurgitation. It starts loudly at S2 after the aortic/pulmonary valves have closed and then diminishes (decrescendos) during diastole as the pressures between the peripheral circulation and the cardiac chambers start to equalise. Look for “the absence of silence” in diastole when auscultating. It is said that you can mimic the sound by whispering the letter “R” out loud.
Mid-diastolic murmur: this begins later in diastole (i.e. after S2) and may be brief or continue up till S1. It is due to turbulent flow through one of the narrowed atrioventricular (tricuspid or mitral) valves, most commonly due to mitral stenosis (although this condition is now rare in developed countries as it was almost invariably due to rheumatic heart disease). It is low pitched and therefore best heard with the bell.
Austin-Flint diastolic murmur: this occurs in severe aortic regurgitation and is said to represent “fluttering” of the mitral valve leaflets during diastole as they are hit by the aortic regurgitant jet of blood.
Graham-Steele diastolic murmur: this specifically refers to a murmur that occurs in mitral stenosis, though a little confusingly not related to the mitral valve itself! When mitral stenosis is severe enough to cause significant pulmonary hypertension, dilatation of the pulmonary artery occurs. This in turn leads to stretch of the pulmonary valve annulus, leading to pulmonary regurgitation (PR). The audible PR is the Graham-Steele murmur.